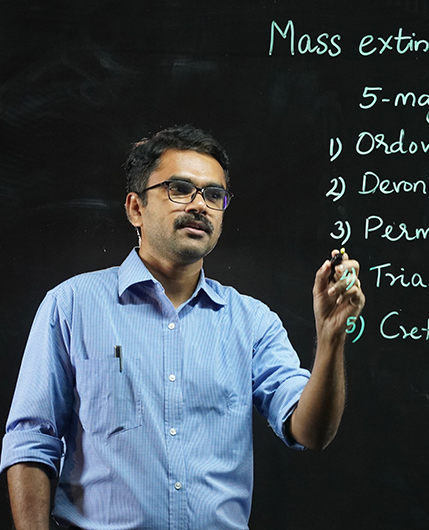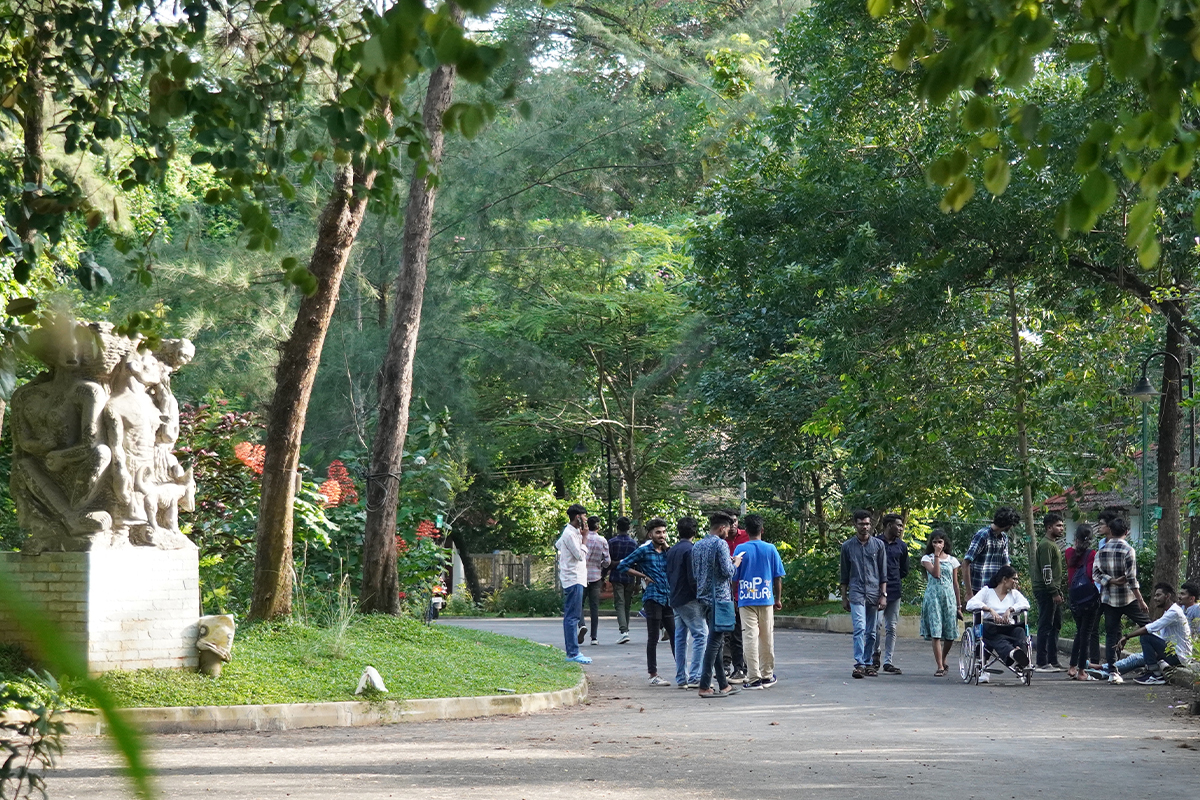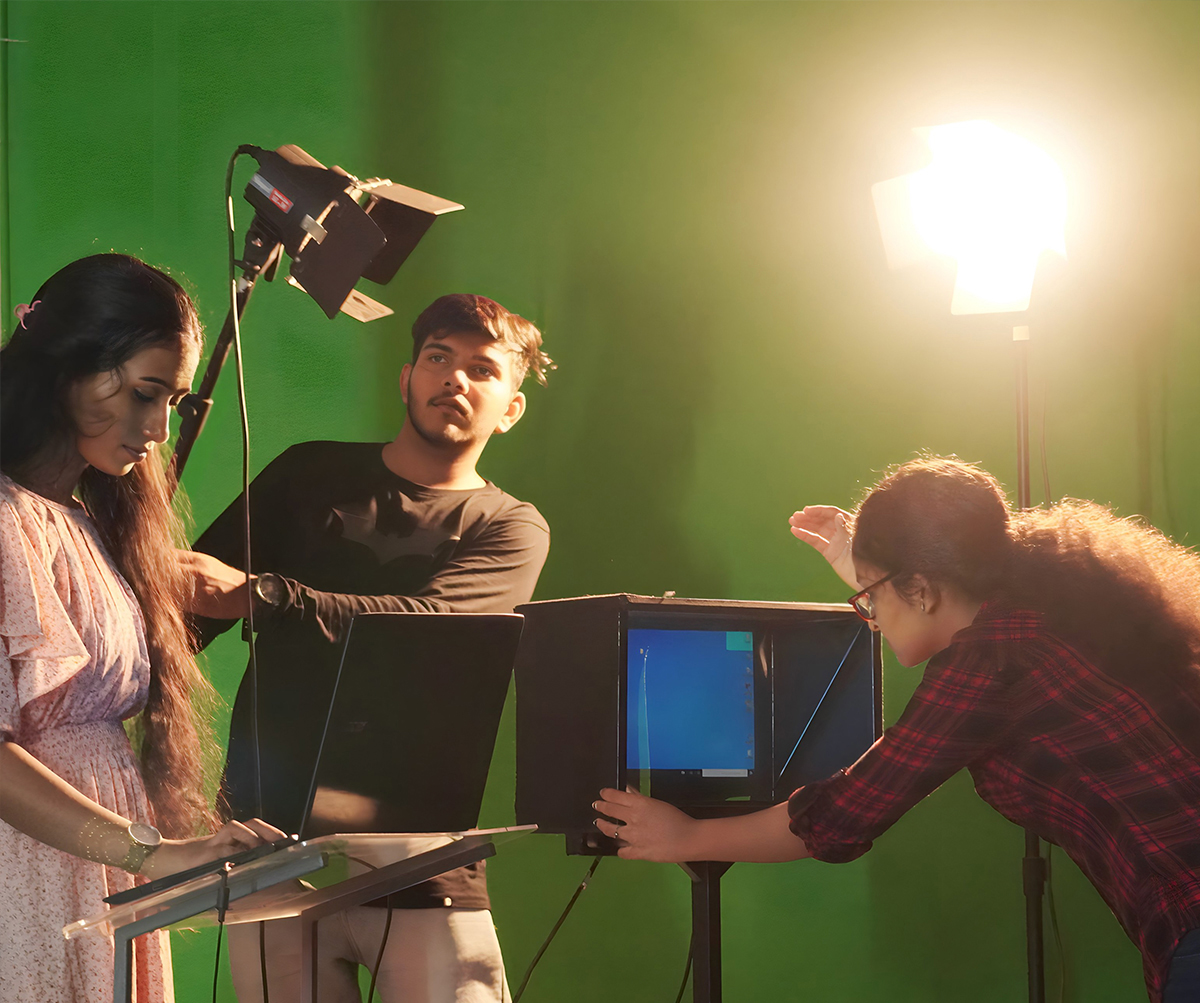March 27, 2024
March 27, 2024
March 27, 2024
CMS News
March 27, 2024
Events
Examinations
CMS Highlights
January 21, 2024
CMS Highlights
CMS Highlights
Innovation, Consultancy Services
and Collaborations
Innovation, Consultancy Services
and Collaborations

This is an earth-friendly story
Sustainability fits well
Sustainability fits well

Leading in the interest of a balanced ecosystem, the campus preserves and maintains the robust functions of the flora and fauna that hosts the community. From birds and insects, to bats and squirrels, the trees provide a canopy for many species of wildlife.
5 Key Points

Air
CMS, is the habitat of 560 species of plants among which around 70 are endangered species. Hence, the first phase of the re-wild project was planting new varieties, around 400 new trees and plants were brought into the campus, out of which 200 are new species. The trees and plants purify the air and rejuvenate the campus.

Soil
The dry leaves compost for effective waste management is one of the most rewarding green practices as once decomposed, they are extremely beneficial to the soil. We thereby promote organic farming in the campus over an area of nearly 30 acres.

Energy
The continued appropriation of nature through sustainable projects like utilising the solar energy to run 33 percent of the entire campus is a potentially compatible use of the natural resource.

Water
The college well is a perennial source of water and provides enough drinking water for more than 3000 people in the community.

Digital
Mastering digital transformation through new initiatives to further advance sustainability in IT solutions.
Awards &
Achievements

NAAC re-accredited with “A” grade
NAAC re-accredited with “A” grade

UGC
Heritage Status
UGC
Heritage Status

Principal’s Message
Pillars of Strength: Heritage, Unique Campus Design, and Sustainability
Pillars of Strength: Heritage, Unique Campus Design, and Sustainability
Our college is anchored on three fundamental pillars—our rich heritage, unique campus design, and unwavering commitment to sustainability. These pillars are not mere components but interconnected facets that shape the character and essence of CMS College Kottayam.
– Prof. Varghese C Joshua.
Watch the latest broadcast from CMS
Watch the latest broadcast from CMS
Direct from CMS
CMS YouTube
Channel
CMS YouTube
Channel
The CMS College Youtube Channel encompasses the activities of the college catalogued into several playlists. A wide range of engaging classes by eminent faculty members with the aid of the light board to stimulating interactions with great minds and college news are the highlights of the channel.
The CMS College Youtube Channel encompasses the activities of the college catalogued into several playlists. A wide range of engaging classes by eminent faculty members with the aid of the light board to stimulating interactions with great minds and college news are the highlights of the channel.